Verein zur Bekämpfung chronischer Infektionskrankheiten e.V.
COMPANY PROFILE
ISB Ion Source & Biotechnologies-Srl
Location: Milano (I)
Established: 2015
PAST MILESTONES
1.9M€ invested since 2006:
2006-2010: Research
2010-2015: Technology development and patents
2016-2017: Clinincal tests
ROADMAP
2019: Samples production and certification
2020: Launch on the market
FINANCIAL INFORMATION
Investments required: 700 K€
Use of funds:
20% Industrialisation
60% Scale-up production
20% Marketing
Turnover:
140 K€ in 2020, 6.2 M€ in 2023
Jobs created: 20 in 5 years
Financial result:
126% IRR, 6.2 M€ NPV
TEAM
PROBLEM TO SOLVE
Foreseeing the advent of degenerative diseases such as cancer and diabetes before or in the very early stages of insurgency, to prevent their diffusion and reduce the social and financial costs for the community.
SOLUTION/PRODUCT
SANIST technology application kits add a new level to degenerative diseases prevention by introducing the possibility of foreseeing the advent of diseases before or in the very early stages of insurgency. The innovative solution is a precision medicine service based on the use of ISB patented SANIST technology. The technology is based on mass spectrometry and data elaboration and it has been validated on patients by the Multimedica IRCCS clinic laboratory center.
MARKET
The market of disease prevention healthcare kits and related services has a global value of over 400 billion €, growing steeply in EU and USA.
COMPETITORS
Solutions for degenerative diseases prevention found in state-of-the-art are mainly based on physical, e.g., xRay analysis, magnetic resonance, and biological evaluation, e.g., detection of circulating cancer cells.
COMPETITIVE ADVANTAGE
The capability of SANIST technology application kits to perform a rapid Ox Stress screening based on our innovative biomarker panel is a clear advantage on competitors’ solution, enabling better performances at a lower price per analysis.
BUSINESS MODEL
SANIST technology application kits are sold to certified clinical institutes that will provide to the citizens a service for the degenerative diseases’ prevention
GO TO MARKET STRATEGY
A SANIST application kits pre-sale is foreseen in 2022 to test the market and acquire the initial 5 clients, recognised clinical centers that will act as testimonials to scale-up sales in the next years.
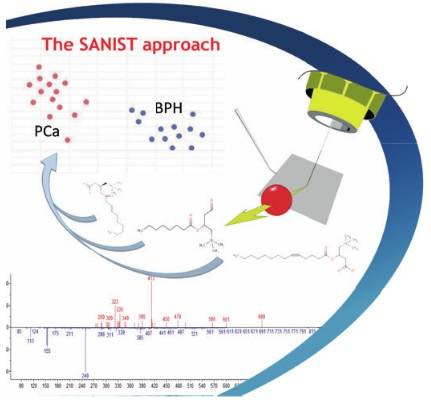
Problem to solve:
Because of the strong increase in the incidence and severity of degenerative disease, such as cancer, diabetes etc, the quality of life of people is strongly at risk in the coming years. In fact, the incidence of diabetes and cancer as leading causes of death is expected to increase by 80% in the next 20 years[1]. This dramatic situation, due to different factors such as the increased life expectancy and the growth of the world population, is likely to lead to a collapse of the healthy system due to the increase in costs and the lack of facilities to assist the high number of unhealthy citizens. Public institutions are implementing extensive programs to control the diffusion of degenerative diseases among the population, based on various approaches. For example, several projects promoted by government institutions are currently on course to prevent the advent of diabetes. Global spending on diabetes was estimated at € 284 billion in 2010 and this expenditure is expected to increase to € 370 billion in 2030.
Nowadays, the methods employed to prevent the diffusion of degenerative diseases are mainly based on the use of questionnaires on the individual’s life-style and on genetic investigation. However, both approaches have been shown to be strongly limited. In fact, they simply indicate a potential predisposition to develop a degenerative disease but cannot identify the presence of metabolic alteration correlated to the effective activation of the degenerative biological process, that will actually lead to the disease symptoms appearance in short time. Thus, new clinical technologies to tackle this problem are strongly needed.
Business opportunity:
There is a huge business opportunity for a new technology, such as the SANIST platform, capable to foresee the advent of degenerative diseases and to provide useful indications of therapeutic prevention. This will increase the citizens’ quality of life in the long term and will reduce costs for healthcare systems, even improving their assets utilization and therapeutic efficiency. The solution will be offered on the growing market of disease prevention healthcare kits and related services, comprising products and methods to monitor the metabolism correlated to degenerative diseases, that constitutes a business opportunity of over 400 billion €, growing steeply in EU and USA.
Solution:
SANIST technology application kits add a new level to degenerative diseases prevention by introducing the possibility of foreseeing the advent of diseases before or in the very early stages of insurgency. This is achieved in two main steps:
The innovative solution can be summarized in a precision medicine service based on the use of ISB’s proprietary SANIST technology (Cristoni et al. Patents n. WO2004034011, WO2007131682 and 102018000004495). The technology is based on mass spectrometry and it has been validated by the Multimedica IRCCS clinic center[2].
The SANIST platform structure (Figure 1) consists of:
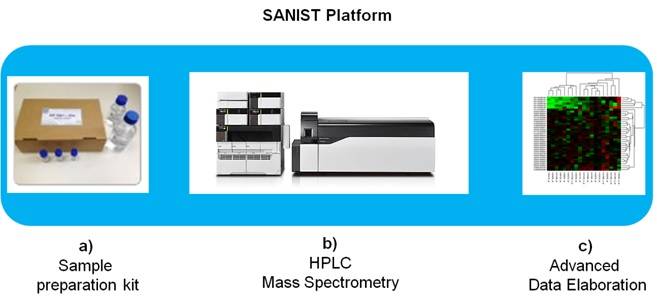
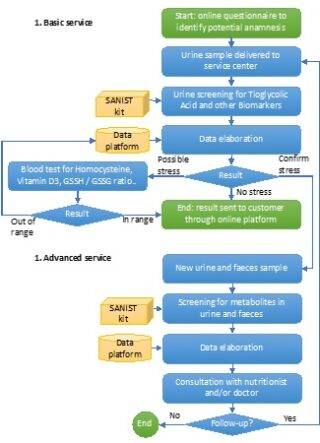
The SANIST solution will be provided as a service by certified clinical centres applying the process shown in Figure 2. The process is based on two main services, both provided through the same integrated platform:
The oxidative stress (OX) has been shown to be propaedeutic to the development of different degenerative diseases. OX will be evaluated in two steps: 1) a first screening step on urines, that are easy to collect, to be massively extended to the entire population of patients and 2) a second confirmation step on blood samples. The urine samples will be sent to the clinical centre. Here they will be screened using the SANIST technology application kits to quantify the urinary thioglycolic acid level (a well-known marker of oxidative stress so far only applied in work medicine) together with a set of biomarkers correlated to cancer and degenerative diseases development or progression.
Patients found positive to oxidative stress test will be investigated for the potential presence of intestinal dysbiosis so to ascertain the presence of intestinal bacteria able to activate biological mechanisms leading to oxidative stress. SANIST application kit will be employed to correlate intestinal dysbiosis with metabolic molecular disorder for prevention and therapy follow up purposes (step 3). It is so possible to identify the toxins by means of specific database search and connect them to the pathogenic intestinal bacteria. Clinical centre experts (e.g., nutritionist) will use the provided information to define specific therapeutic protocols to equilibrate the intestinal dysbiosis and prevent or treat the degenerative diseases associated with the dysbiosis.
State-of-the-art:
Solutions for degenerative diseases prevention found in state-of-the-art are mainly based on physical and biological evaluation. Physical approaches include mammography, xRay analysis, magnetic resonance and similar methods. Biological approaches are based on biological interaction, e.g., detection of circulating cancer cells. Despite the sensitivity and selectivity of each of these approaches, they are in general not useful for early stage diagnosis of diseases, that are mostly discovered in medium or advanced stages. This is mainly due to the intrinsic technical limits of the employed techniques. The physical methods like xRay and magnetic resonance make use of radiations or electromagnetic fields, detecting their interaction with tissues to discover tumours or other lesions. The main limitation is that a minimal dimension of the lesion area is needed for the detection[3]. Even in this case a minimum number of absorbing molecules are necessary to obtain a defined image, limiting the application of this technology in early stage disease detection and prevention.
Most of the biological approaches employed routinely make use of physical detection technique (e.g.: light absorption or emission) that also exhibits sensitivity and selectivity limits, therefore they are mainly used for metastatic progression evaluation[4].
Finally, the cited physical and biological approaches are essentially devoted to the cancer diagnosis and are not suitable for the detection and prevention of other degenerative diseases like neurological ones (e.g.: multiple sclerosis, lateral amyotrophic sclerosis), autoimmunity etc.
SANIST Vs. State-of-the-art:
The SANIST platform is based on mass spectrometry technology that has seen a strong diffusion, in the clinical world, in the last 10 years. Thanks to its higher selectivity and sensitivity, due to application of the electromagnetic physical phenomenon used in mass spectrometry to detect metabolites charged ions produced by ionization source, Normally, the bacteria present in faeces produce toxins that are absorbed by other bacteria or wasted through faecal material. When an intestinal dysbiosis occurs, an excess of toxins produced by the pathogen bacteria are absorbed in the body and after provide body damages are wasted in the urine. SANIST employs the innovative SACI/ESI application kit ionization, proprietary of ISB, to increase the mass spectrometric sensitivity so to enhance the detection of human or bacteria metabolites correlated to the degenerative diseases. SANIST is thus able to finely detect the human and bacteria metabolism and to connect it to diseases prevention.
With respect to the classical technologies for the detection of bacteria and to the related toxicology SANIST is able to detect the bacteria with higher rapidity and efficiency and in the early stage of the dysbiosis. The usually employed kit used in clinic procedure are able to detect the bacteria only when their grown is advanced and the patient health is compromised. With SANIST it is possible to detect the presence even of few bacteria that will lead to the disease symptoms in the future, so acting at prevention level. No technology before SANIST has been able to achieve this goal. Table 1 shows the comparison between the technology benefits and applicability:
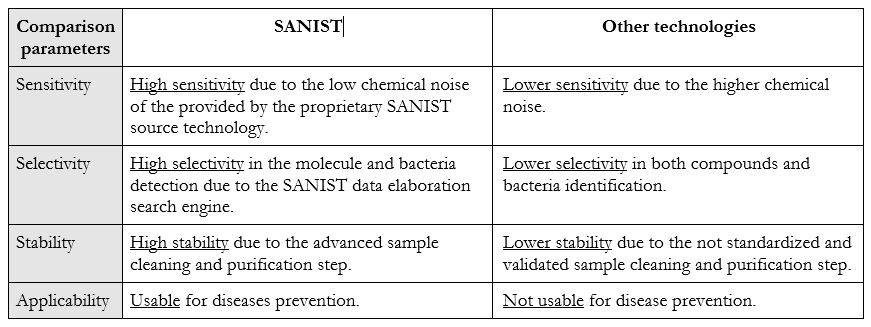
Project uniqueness:
ISB core patented technology, the SANIST application kits, is unique on the market thanks to the capability to evaluate dysbiosis on the basis of the molecular metabolism. To this purpose the urine and faeces samples of the same patient can be analysed to obtain their toxin molecular profiles. The SANIST application kit analysis is capable to find these toxins by combining a urinary and faeces molecular profile. The unique patented porfolio (see section 2.4) is applicable to the prevention and therapy follow-up emerging market. SANIST is the unique technology capable to simultaneously detect the diseases and to connect them to intestinal dysbiosis toxicology, so to understand the disease origin and apply the correct therapy.
Company past milestones:
Starting from 2006 relevant research investments, 1.9 M€ in total, have been sustained by ISB srl to develop the SANIST technology and the related application kits for intestinal dysbiosis. In particular:
Current stage of development:
In its current version the technology has been tested in the relevant clinical environment. The tests have been carried out over two years in collaboration with healthy and unhealthy subjects in collaboration with Multimedica IRCCS clinical centre. More than 100 samples have been analysed between 2016 and 2017 to test the performance of SANIST platform for the prevention of the degenerative diseases. The test published results show that the technology is effective in diseases prevention area[5]. A subject control group of people have been investigated to detect potential dysbiosis activities in early stages. All of them developed the predicted dysbiosis after about three months from the evaluation. Table 2 shows the results achieved in the disease prevention applied analyzing Ox Stress level combined to intestinal dysbiosis evaluation on the 100 selected subjects. On the basis of the literature data associated to the Ox Stress and the bacteria was also possible to predict the potential diseases associated to the achieved data. The percentage % of subjects that will develop the disease within one year from the analysis is also reported in Table 2.
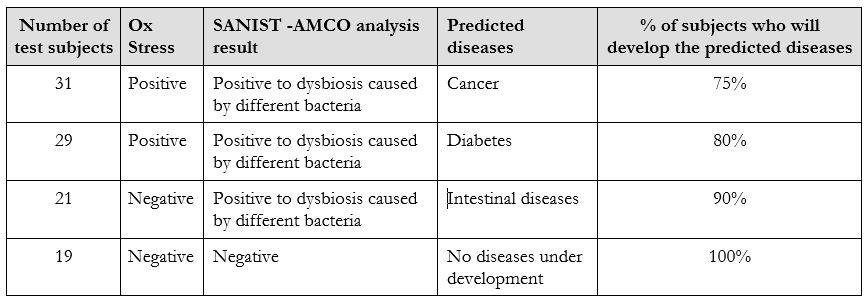
As it shown in Table 2, the dysbiosis data has been correlated to the Ox Stress evaluation based on the dosage of three recognized molecule: homocysteine, Vitamin D and Urinary thiodiglycolic acid[5]. The efficacy in specific diseases prediction after one year from the test was among 75 and 100 %. Actually, no technology present on the market was able to obtain a similar level of diseases predictivity in the prevention stage.
Objectives of the feasibility study:
The aim of the proposed study is to assess the economic and technical feasibility of a business plan to bring the SANIST platform on the market. The Phase 1 project will elaborate a detailed business plan achievable within the duration of the project (6 months), related to detailed measurable specific objectives listed below:
OBJ 1: Industrialization and production scale-up planning: to assess the requirements for all the activities, resources and supplier partners necessary to industrialise the current SANIST platform kits and services; to plan in detail the required investments to scale-up production capacity in accordance with the 5 years sales forecast.
OBJ 2: Product test and certification planning: to define the product tests and procedures required for CE certification for product distribution in the target international markets, and to plan the related activities and costs.
OBJ 3: Definition of the IPR strategy: to carry out a patentability analysis on international, national and local level with the aim to apply for further protection of the SANIST technology on the various target markets; to define the strategy for further protect valuable knowledge of the product on international level.
OBJ 4: Market analysis and commercialization planning: to assess the existing and potential market (size and growth) on EU and global scale; to identify the product promotion targets and channels; to identify the required activities, resources and related costs; to search for industrial partners for the target international countries (e.g., international distributors) and to define agreements with them.
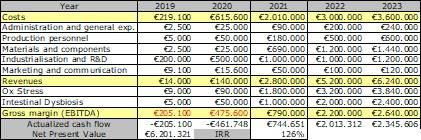
OBJ 5 – Consolidate all the information collected into a business plan: quantification of the revenues and costs based on the detailed market analysis and on the cost analysis of SANIST platform production and related services; financing requirements and Return On Investment evaluation, by forecasting the cash flow and projecting financial indicators on a 5 years period, and by identifying the planned sources of funding.
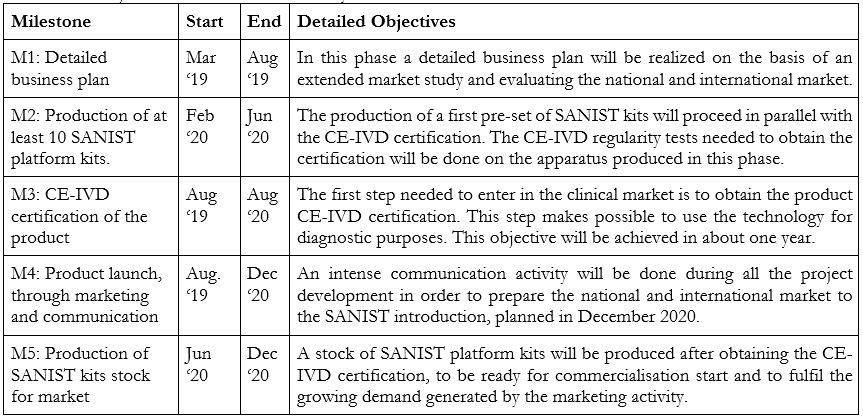
Next Development Steps to commercialize the product:
The main future milestones to be achieved are two:
The detailed objectives and activities necessary to fulfil these milestones are described in the Table below:
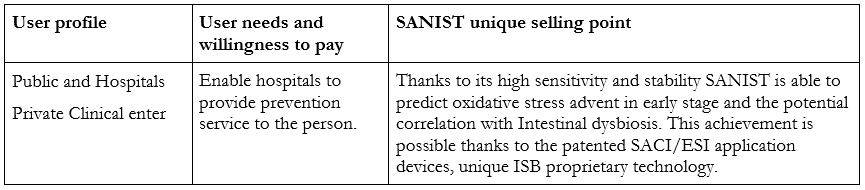
Relation with the users:
The SANIST technology has been used for different research pilot studies in collaboration with Multimedica (multimedica.it). The technology has recently been installed in the laboratory of Desio, Monza Hospitals that has collaborated with ISB and Multimedica IRCCS Hospital in the R&D activities.
Target Market:
The global preventive healthcare technologies and services market is expected to reach $ 432.4 billion by 2024[6]. Key factors driving the market expansion include the growing awareness about disease prevention and therapeutic follow up based on personalized medicine, which enhance the quality of life, lifespan, and minimize the possible healthcare spending. Growth of the market is also fuelled by the factors such as reducing birth rate that is resulting in increase of geriatric population, which is more prone to the chronic diseases.
North America has been identified as the biggest and quickest developing market between 2014 to 2020 for preventive healthcare technologies and services. The steady repayment strategies, the development of big data innovation, the high mindfulness about preventive medicinal services advances and the emergence of real players in this market are the main factor supporting the development of this market in North America. Asia-Pacific and Rest of the World are expected to be potential growing markets in coming years, with the present market infiltration evaluated to be low[7].)
The prevention and therapy follow up market can be mainly segmented and focalized on the following diseases:
Competitors analysis:
The most common competitors’ solutions are these based on mass spectrometry. SANIST technology kits are based on mass spectrometry too, but their unique patented features allow to increase performance in terms of data type, quality and elaboration. Moreover, the analysis time is lower with respect to the other mass spectrometric platform increasing both throughput and productivity. The main competitors of SANIST are mainly US-based companies:

Competitive advantage and positioning strategy
The capability of SANIST technology application kits to perform a rapid Ox Stress screening based on our innovative biomarker panel is a clear advantage on competitors’ solution, enabling better performances at a lower price per analysis (Figure 2). This will make it possible to achieve a rapid and progressive business growth.
On the other hand, SANIST can be positioned on the market as a complementary solution, providing added value on most common diagnostic methods. Indeed, our application kit makes it possible to correlate the oxidative stress with the intestinal dysbiosis so to find the diseases origin and apply a correct therapy. The intestinal dysbiosis data are obtained by evaluating the circulating toxins emitted from the microbioma. The test is fully compatible to that employed in clinics today for diagnostic purposes due to the fact that it is employed in the prevention step. In fact, the subjects that are identified as likely to develop degenerative diseases will be monitored by means of the classical clinical employed approaches based on the previously described physical and biological diagnostic methods.
ISB business model is based on commercialization of patented SANIST technology application kits to be sold to certified clinical institutes that will provide to the citizens a service for the degenerative diseases’ prevention. ISB will interact with three main types of stakeholders: 1) Clinical centres, that will provide the service following certified protocols for the technology applications, 2) ICT providers specialised in bioinformatics to provide the data elaboration and related application services and 3) Research centres to constantly improve the SANIST product performance. The business model is completely open so new partners (clinical, research and bioinformatics center) can be progressively added for the business to grow and expand the customers base.
ISB has already long-term relationships with key partners in three categories of stakeholders:
Relationships have also been established with other important stakeholders, in particular with Waters corporation, an international distributor providing Liquid Chromatography Mass Spectrometric technologies used in the SANIST technology. In 2012 waters has signed an agreement with ISB for obtaining the exclusivity in the commercialization of the SANIST core patented technology (SACI and USIS ionization sources). €277.000 have been payed as a front fee by Waters to ISB to obtain the patent rights. Waters has given to ISB a retro-exclusivity for the commercial use of the technology as application kits, a key milestone that led to the SANIST technology application kit.
The revenue model is based on three main elements: i) the SACI/ESI ionization source technology, to increase the performances of existing mass spectrometers, will be installed for free; ii) the data elaboration and management platform will be offered for a 1.000€ fee, including full assistance and support services; iii) the sample preparation kit will be provided at a price of 80 €/analysis for intestinal dysbiosis and 40€/analysis for Oxidative stress panel.
The plan for commercialisation includes the following steps: i) promote a SANIST application kits pre-sell (February-June 2020) to test the market; ii) installation of the kit technology in at least 5 recognized clinical reference centers; iii) marketing and communication strategy focalized on testimonials from the reference centers selected in step ii), through various channels: social media, interviews and articles on sectorial magazines and scientific journals; iv) creation of a network of agents to extend and increase market penetration.
This business model could be expanded at national and international market creating reference clinical centers to prevent the degenerative diseases and reducing the clinical therapeutic cost. It will be necessary to open an agency in each state most of all for product assistance and commercialization.
Company Description:
ISB is a solid company active by 10 years that has invested the majority of the capital on the SANIST platform application kits development. It will proceed in co-finance the project through to the revenue obtained by its business. SANIST technology is an evolution of the SACI and SACI/ESI ISB patented technologies (Cristoni et al. Patent n. US20060145089, EP1855306A1). The technology has been developed with an investment of €1.900.000. €1.200.000 had been obtained through Italian Government Grants, while €700.000 were directly co-financed by ISB.
Growth potential: the table below summarises SANIST financial plan over 5 years since the start of the Phase 1 project in 2019. The project represents an exceptional growth opportunity for the company. Considering the total investment required, the project has a Net Present Value of over 6 M€ euro and an Internal Return Rate of about 120% over 5 years. 20 new jobs will be created.
Funding requirement: The overall funding requirement is around 700 K€, including 980 K€ investments for the items in the detailed activities plan in Section 1.2. The sources of funding will include: shareholders capital, bank loans, EU and national grants.
Legal framework: Product CE-IVD certification is needed to operate in the selected clinical centers where it will be commercialized. CE-IVD certification will be obtained in the first 12 month of Phase 2 activities.
IPR Items: ISB owns three international patents on the core technology components: SACI, Basic prevention and treatment monitoring service (WO2004034011); USIS, Advanced prevention and treatment monitoring service (WO2007131682), CRAMS, Biomarker profile based technology for disease prevention (102018000004495).
The project head will be led by Dr Simone Cristoni, CEO of ISB srl. The team will include Martina Larini,Silvia Mendoza (marketing), Angela Perego (finance) and Karim Amaya (finance).

[1] Sources: World Health Organization and „Long Term in Diabetes“ 2017 report.
[2] Albini A. et al. SANIST: a rapid mass spectrometric SACI/ESI data acquisition and elaboration platform for verifying potential candidate biomarkers. Rapid Commun Mass Spectrom. 2015 Oct 15;29(19):1703-10. doi: 10.1002/rcm.7270. The paper comunication has gained high interest in the scientific comunity and SANIST has been inserted on the cover of Rapid Communications in Mass Spectrometry peer reviewed scientific journal (Attachment 2).
[3] Preston RJ et al. J Radiol Prot. 2013 Sep;33(3):573-88; Goldman M. West J Med. 1982 Dec;137(6):540-7, Ibrahim MA. et al. StatPearls. Treasure Island (FL): StatPearls Publishing; 2018 Jan-2018 Jan 25 or ncbi
[4] Gutierrez-Juarez G. et al. Lasers Surg Med. 2010 Mar;42(3):274-81
[5] Albini et al. Rapid Commun Mass Spectrom. 2015 Oct 15;29(19):1703-10; Cristoni S. et al. J Mass Spectrom. 2017 Jan;52(1):16- 21
[6] Leach NV. et al. Eur J Intern Med. 2014 Oct;25(8):762-7; Zhang HQ. Nutrition. 2014 Sep;30(9):1040-4; Puccio G. et al. Rapid Commun Mass Spectrom. 2013 Feb 15;27(3):476-80
[7] Grandviewresearch – press release global preventive healthcare technologies and services market
[8] Information extracted from Preventive Healthcare Technologies market study
[9] Grandviewresearch press release – global cancer tumor profiling ctp market
[10] Globaldata – type-2 diabetes market double 64 billion 2026