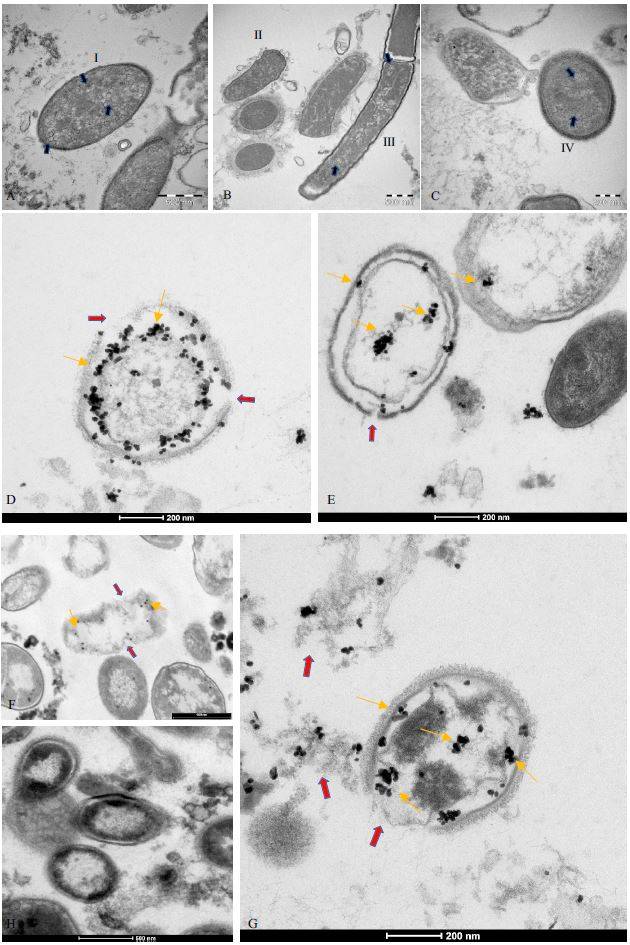
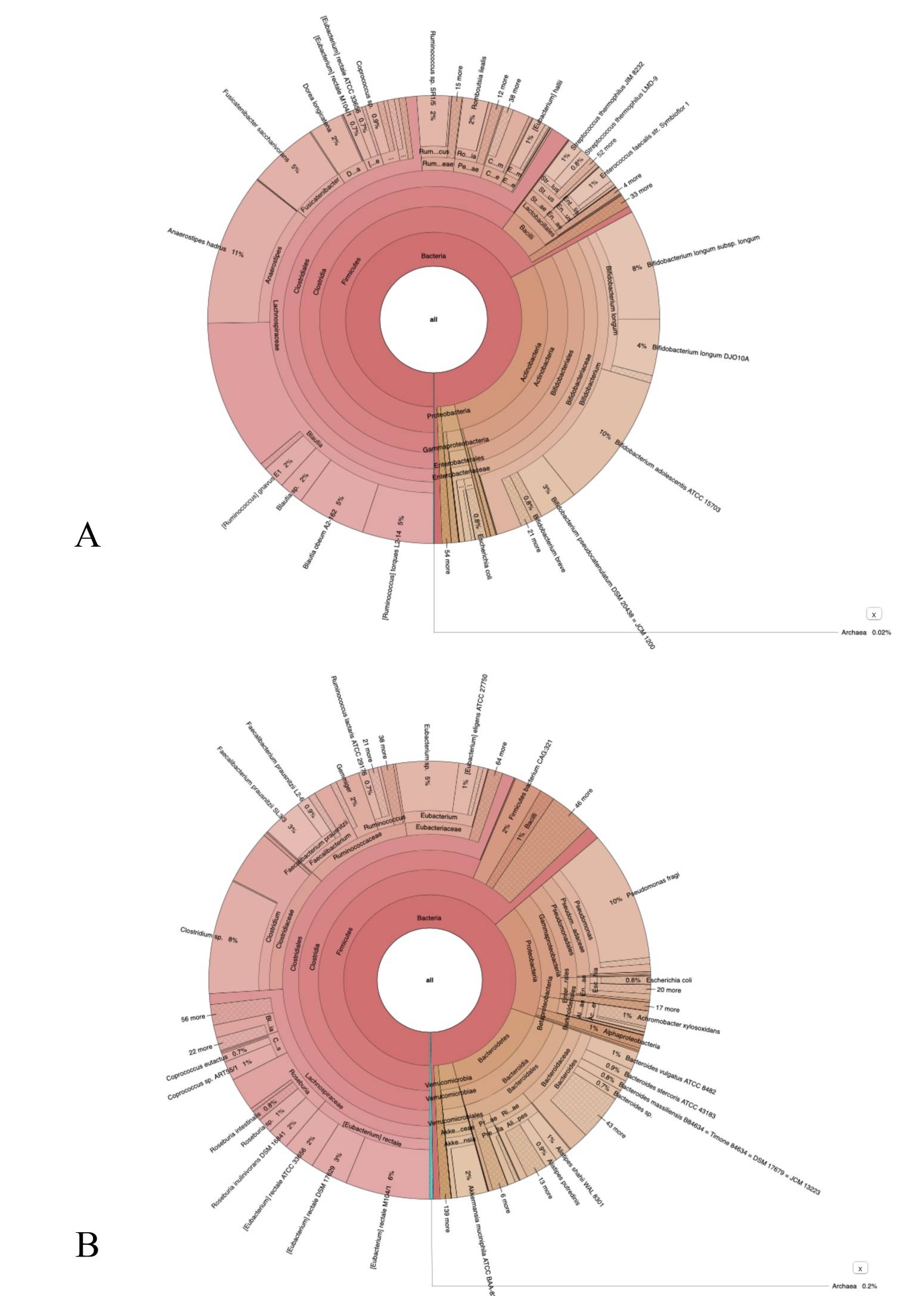
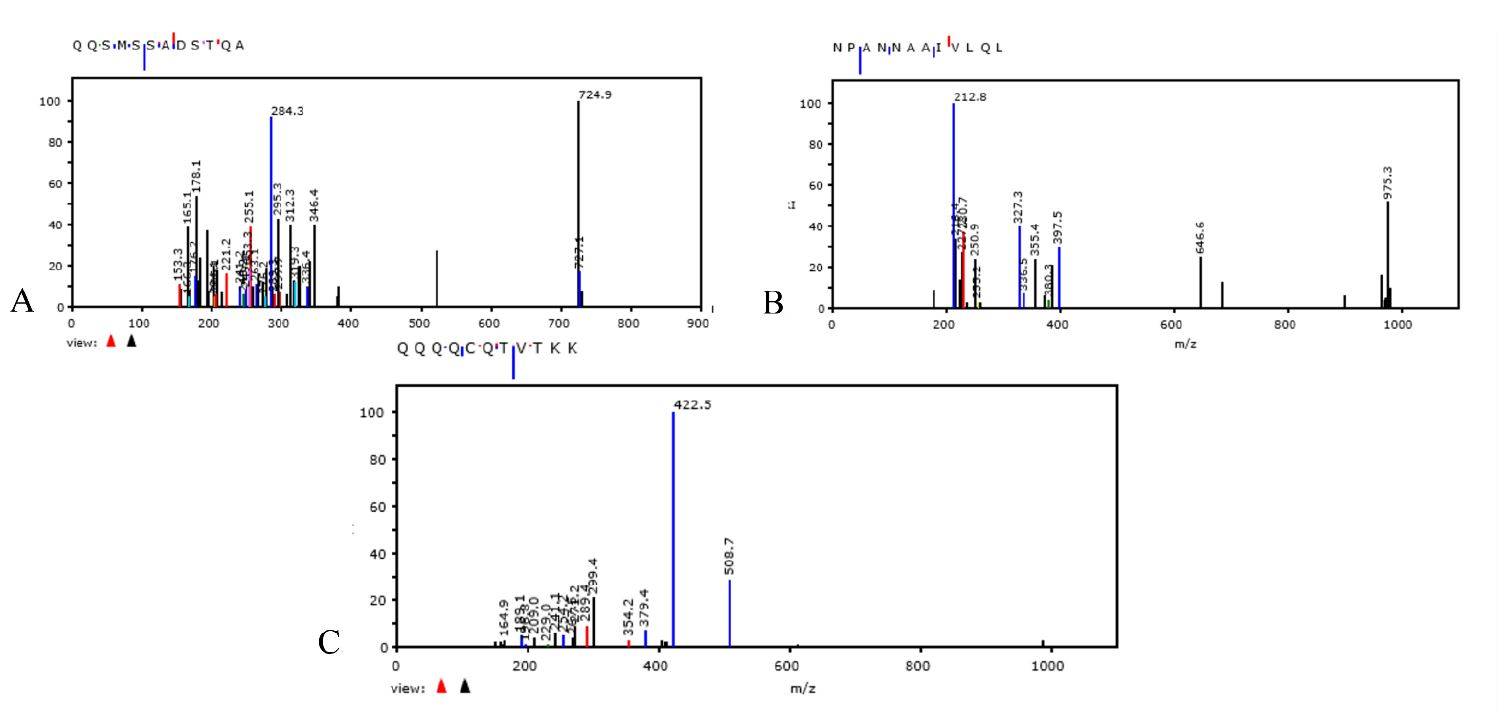
WHO Coronavirus (COVID-19) Dashboard. Available online:
https://www.statista.com/statistics/1194934/number-of-covidvaccine-doses-administered-by-county-worldwide (accessed on 7 April 2021).
2. Chung, M.K.; Karnik, S.; Saef, J.; Bergmann, C.; Barnard, J.; Lederman, M.M.; Tilton, J.; Cheng, F.; Harding, C.V.; Young, J.B.; et al. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 2020, 58, 102907. [
CrossRef] [
PubMed]
3. Whittaker, G.R. SARS-CoV-2 spike and its adaptable furin cleavage site. Lancet Microbe 2021, 2, e488–e489. [
CrossRef]
4. Ge, X.Y.; Li, J.L.; Yang, X.L.; Chmura, A.A.; Zhu, G.; Epstein, J.H.; Mazet, J.K.; Hu, B.; Zhang, W.; Peng, C.; et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013, 503, 535–538. [
CrossRef] [
PubMed]
5. Zajac, V.; Matelova, L.; Liskova, A.; Mego, M.; Holec, V.; Adamcikova, Z.; Stevurkova, V.; Shahum, A.; Krcmery, V. Confirmation of HIV-like sequences in respiratory tract bacteria of Cambodian and Kenyan HIV-positive pediatric patients. Med. Sci. Monit. 2011, 17, CR154–CR158. [
CrossRef]
6. Brogna, C.; Cristoni, S.; Petrillo, M.; Querci, M.; Piazza, O.; Van den Eede, G. Toxin-like peptides in plasma, urine and faecal samples from COVID-19 patients. F1000Research 2021, 10, 550. [
CrossRef]
7. Petrillo, M.; Brogna, C.; Cristoni, S.; Querci, M.; Piazza, O.; Van den Eede, G. Increase of SARS-CoV-2 RNA load in faecal samples
prompts for rethinking of SARS-CoV-2 biology and COVID-19 epidemiology. F1000Research 2021, 10, 370. [
CrossRef]
8. Relationship between the Gut Microbiome and Diseases, Including COVID-19. Available online:
https://ec.europa.eu/jrc/en/news/covid-19-and-our-gut-microbiome-evidence-close-relationship (accessed on 20 October 2021).
9. Wang, H.; Wang, H.; Sun, Y.; Ren, Z.; Zhu, W.; Li, A.; Cui, G. Potential Associations between Microbiome and COVID-19. Front.
Med. 2021, 8, 785496. [
CrossRef]
10. Haiminen, N.; Utro, F.; Seabolt, E.; Parida, L. Functional profiling of COVID-19 respiratory tract microbiomes. Sci. Rep. 2021, 11, 6433. [
CrossRef]
11. Honarmand Ebrahimi, K. SARS-CoV-2 spike glycoprotein-binding proteins expressed by upper respiratory tract bacteria may prevent severe viral infection. FEBS Lett. 2020, 594, 1651–1660. [
CrossRef]
12. Dragelj, J.; Mroginski, M.A.; Ebrahimi, K.H. Hidden in Plain Sight: Natural Products of Commensal Microbiota as an Environmental Selection Pressure for the Rise of New Variants of SARS-CoV-2. Chembiochem 2021, 22, 2946–2950. [
CrossRef]
13. Podlacha, M.; Grabowski, Ł.; Kosznik-Kaw´snicka, K.; Zdrojewska, K.; Stasiłoj´c, M.; W˛egrzyn, G.; W˛egrzyn, A. Interactions of Bacteriophages with Animal and Human Organisms-Safety Issues in the Light of Phage Therapy. Int. J. Mol. Sci. 2021, 22, 8937. [
CrossRef] [
PubMed]
14. Shang, C.; Liu, Z.; Zhu, Y.; Lu, J.; Ge, C.; Zhang, C.; Li, N.; Jin, N.; Li, Y.; Tian, M.; et al. SARS-CoV-2 Causes Mitochondrial Dysfunction and Mitophagy Impairment. Front. Microbiol. 2022, 12, 780768. [
CrossRef] [
PubMed]
15. Kohda, K.; Li, X.; Soga, N.; Nagura, R.; Duerna, T.; Nakajima, S.; Nakagawa, I.; Ito, M.; Ikeuchi, A. An In Vitro Mixed Infection Model With Commensal and Pathogenic Staphylococci for the Exploration of Interspecific Interactions and Their Impacts on Skin Physiology. Front. Cell Infect. Microbiol. 2021, 11, 712360. [
CrossRef] [
PubMed]
16. Kameli, N.; Borman, R.; Lpez-Iglesias, C.; Savelkoul, P.; Stassen, F.R.M. Characterization of Feces-Derived Bacterial Membrane Vesicles and the Impact of Their Origin on the Inflammatory Response. Front. Cell Infect. Microbiol. 2021, 11, 667987. [
CrossRef] [
PubMed]
17. Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell 2020, 11, 771–775. [
CrossRef]
18. Cristoni, S.; Rubini, S.; Bernardi, L.R. Development and applications of surface-activated chemical ionization. Mass Spectrom. Rev. 2007, 26, 645–656. [
CrossRef]
19. Chen, X.; Yu, L.; Steill, J.D.; Oomens, J.; Polfer, N.C. Effect of peptide fragment size on the propensity of cyclization in collisioninduced dissociation: Oligoglycine b(2)-b(8). J. Am. Chem. Soc. 2009, 131, 18272–18282. [
CrossRef]
20. Bertrand, K. Survival of exfoliated epithelial cells: A delicate balance between anoikis and apoptosis. J. Biomed. Biotechnol. 2011, 2011, 534139. [
CrossRef]
21. Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [
CrossRef]
22. Zajac, V.; Mego, M.; Martinický, D.; Stevurková, V.; Cierniková, S.; Ujházy, E.; Gajdosík, A.; Gajdosíková, A. Testing of bacteria isolated from HIV/AIDS patients in experimental models. Neuro Endocrinol. Lett. 2006, 27 (Suppl. S2), 61–64.
23. Xu, H.; Wang, X.; Veazey, R.S. Mucosal immunology of HIV infection. Immunol. Rev. 2013, 254, 10–33. [
CrossRef] [
PubMed]
24. Diallo, B.; Sissoko, D.; Loman, N.J.; Bah, H.A.; Bah, H.; Worrell, M.C.; Conde, L.S.; Sacko, R.; Mesfin, S.; Loua, A.; et al. Resurgence of Ebola Virus Disease in Guinea Linked to a Survivor With Virus Persistence in Seminal Fluid for More Than 500 Days. Clin. Infect. Dis. 2016, 63, 1353–1356. [
CrossRef]
25. Cheung, K.S.; Hung, I.F.N.; Chan, P.P.Y.; Lung, K.C.; Tso, E.; Liu, R.; Ng, Y.Y.; Chu, M.Y.; Chung, T.W.H.; Tam, A.R.; et al. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology 2020, 159, 81–95. [
CrossRef] [
PubMed]
26. Brogna, B.; Brogna, C.; Petrillo, M.; Conte, A.M.; Benincasa, G.; Montano, L.; Piscopo, M. SARS-CoV-2 Detection in Fecal Sample from a Patient with Typical Findings of COVID-19 Pneumonia on CT but Negative to Multiple SARS-CoV-2 RT-PCR Tests on Oropharyngeal and Nasopharyngeal Swab Samples. Medicina 2021, 57, 290. [
CrossRef] [
PubMed]
27. McIntosh, K.; Kapikian, A.Z.; Turner, H.C.; Hartley, J.W.; Parrott, R.H.; Chanock, R.M. Seroepidemiologic studies of coronavirus infection in adults and children. Am. J. Epidemiol. 1970, 91, 585–592. [
CrossRef]
28. Ma, Y.; Zhang, Y.; Liang, X.; Lou, F.; Oglesbee, M.; Krakowka, S.; Li, J. Origin, evolution, and virulence of porcine deltacoronaviruses in the United States. MBio 2015, 6, e00064. [
CrossRef]
29. Sabin, A.B.; Ward, R. The natural history of human poliomyelitis: II. Elimination of the virus. J. Exp. Med. 1941, 74, 519–529. [
CrossRef]
30. Drinker, P.; Shaw, L.A. An apparatus for the prolonged administration of artificial respiration: I. A design for adults and children.
J. Clin. Investig. 1929, 7, 229–247. [
CrossRef]
31. Sabin, A.B. Pathogenesis of poliomyelitis; reappraisal in the light of new data. Science 1956, 123, 1151–1157. [
CrossRef]
32. Sabin, A.B. Behaviour of Chimpanzee-avirulent Poliomyelitis Viruses in Experimentally Infected Human Volunteers. Br. Med. J.
1955, 2, 160–162. [
CrossRef]
33. Sabin, A.B. Present status of attenuated live virus poliomyelitis vaccine. Bull. N. Y. Acad. Med. 1957, 33, 17–39. [
CrossRef] [
PubMed]
34. Sabin, A.B. Oral Poliovirus Vaccine: Recent results and recommendations for optimum use. R. Soc. Health J. 1962, 82, 51–59. [
CrossRef] [
PubMed]
35. Likar, M.; Bartley, E.O.; Wilson, D.C. Observations on the interaction of poliovirus and host cells in vitro. III. The effect of some bacterial metabolites and endotoxins. Br. J. Exp. Pathol. 1959, 40, 391–397. [
PubMed]
36. Petrillo, M.; Querci, M.; Brogna, C.; Ponti, J.; Cristoni, S.; Markov, P.V.; Valsesia, A.; Leoni, G.; Benedetti, A.; Wiss, T.; et al. Evidence of SARS-CoV-2 bacteriophage potential in human gut microbiota. F1000Research 2022, 11, 292. [
CrossRef]
37. Liu, J.; Liu, S.; Zhang, Z.; Lee, X.; Wu, W.; Huang, Z.; Lei, Z.; Xu, W.; Chen, D.; Wu, X. Associationbetween the nasopharyngealmicrobiome and metabolome in patients with COVID-19. Synth. Syst. Biotechnol. 2021, 6, 135–143. [
CrossRef]